Buy OxyContin online without prescription
Product Details Table
| Specification Category | Details |
|---|---|
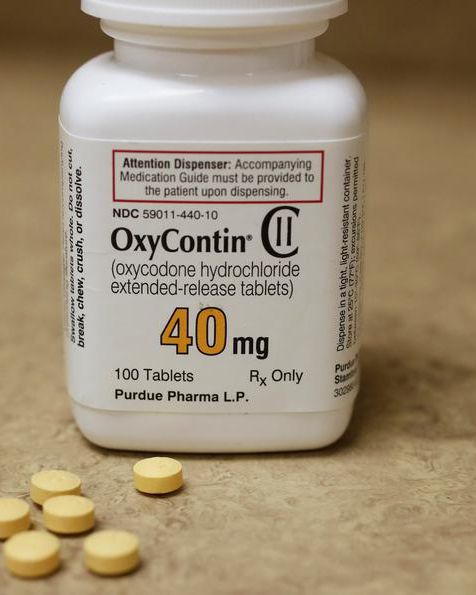
| Brand | OxyContin |
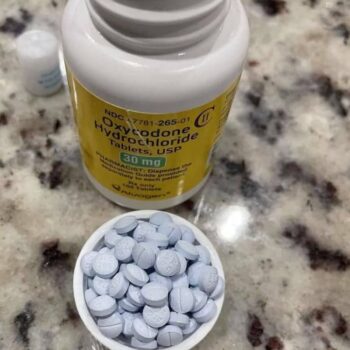
| Composition | Oxycodone hydrochloride |
| Concentration | Available in multiple strengths (10mg, 15mg, 20mg, 30mg, 40mg, 60mg, 80mg oxycodone HCl) |
| Duration of Effects | Designed for 12-hour controlled release |
| Indications | Management of severe, persistent pain requiring continuous opioid therapy |
| Administration | Oral, swallowed whole with adequate water. Do not crush, chew, or dissolve. |
| Shelf Life | 24 months from manufacturing date when stored properly |
| Storage Conditions | 20-25°C (68-77°F); protect from moisture and light; secure controlled storage |
| Package Contents | Manufacturer-sealed bottles with child-resistant closures |
| Country of Origin | United States (manufactured in licensed facilities) |
| Licensing | FDA-approved with REMS requirements; DEA Schedule II controlled substance |
| Safety Notes | Black Box Warning for addiction, abuse, misuse, life-threatening respiratory depression, neonatal opioid withdrawal syndrome, risks from concomitant use with benzodiazepines or CNS depressants |
$160.00 – $900.00
Buy it nowProduct Description
OxyContin is a pharmaceutical formulation containing oxycodone hydrochloride as its primary active component. This extended-release medication belongs to the opioid analgesic class and is specifically engineered for sustained pain management. The product features a proprietary CONTIN® delivery system that gradually releases medication over 12 hours, providing consistent plasma concentrations for stable pain control.
Mechanism of Action
The therapeutic effect occurs through oxycodone’s action as a full opioid agonist, primarily binding to mu-opioid receptors in the central nervous system. This interaction modifies pain perception and emotional response to discomfort. The extended-release matrix utilizes a patented delivery mechanism that controls dissolution and absorption rates, ensuring predictable pharmacokinetics when administered as directed.
Benefits and Therapeutic Advantages
Patients requiring around-the-clock opioid therapy benefit from the consistent analgesic effect, which helps maintain functional capacity and quality of life. The controlled-release formulation reduces dosing frequency compared to immediate-release alternatives, potentially improving adherence to pain management regimens. Clinical studies demonstrate reliable pain relief for extended periods when used according to established medical guidelines.
Safety and Responsible Usage Considerations
This medication carries significant risks including addiction, abuse, misuse, life-threatening respiratory depression, and accidental exposure. It should only be used under close medical supervision with appropriate patient monitoring. The U.S. Food and Drug Administration (FDA) requires a Risk Evaluation and Mitigation Strategy (REMS) program for all opioid analgesics. Patients should be educated about proper storage, disposal, and recognition of overdose symptoms. Concurrent use with benzodiazepines or other CNS depressants increases risk of profound sedation, respiratory depression, and potential fatality.
Storage and Stability Parameters
Maintain original packaging at controlled room temperature (20-25°C or 68-77°F) with brief excursions permitted between 15-30°C (59-86°F). Protect from excessive moisture and direct light. Keep securely locked away from unauthorized persons, particularly children, pets, and individuals without legitimate medical need. Unused medication should be disposed through authorized take-back programs when possible.
Composition and Formulation Details
Each tablet contains oxycodone hydrochloride as the active pharmaceutical ingredient. Inactive components vary by strength but commonly include cellulose derivatives, stearic acid, and proprietary release-controlling polymers. The formulation is designed to resist extraction for non-medical use while providing consistent therapeutic release when swallowed intact.
Research Applications
In controlled laboratory environments, this product serves as a reference standard for analgesic research, pharmacokinetic studies, and receptor binding assays. Educational institutions utilize it for pharmacological instruction about opioid mechanisms, risk mitigation strategies, and pain management protocols. Regulatory bodies reference it when developing abuse-deterrent formulation standards.
Quality Assurance and Patient Trust
Manufactured under current Good Manufacturing Practices (cGMP) with rigorous quality control testing, each batch undergoes comprehensive analysis for purity, potency, and release characteristics. Healthcare providers worldwide recognize the consistent quality when prescribing this medication for appropriate patients. For comprehensive information about pharmaceutical research materials and educational resources, visit our official portal at Health Aid Pharmacy.
Responsible use requires balancing effective pain management with vigilant risk mitigation. Always consult qualified healthcare professionals for personalized medical advice regarding pain treatment options.
| Price Options 40 & 60mg | 60 TABS, 100 TABS, 300 TABS, 500 TABS |
|---|
| Ingredients | Skimmed milk powder, lactose, vegetable oils (palm, rapeseed, sunflower), palm kernel fat, calcium carbonate, calcium salts of phosphoric acid, vitamin C, ferrous sulphate, stabilizer: L (+) - lactic acid, vitamin D, riboflavin , vitamin A, potassium iodate. | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Nutritional values |
|
||||||||
| Available for age | 6-12 months, 12-24 months |














There are no reviews yet.