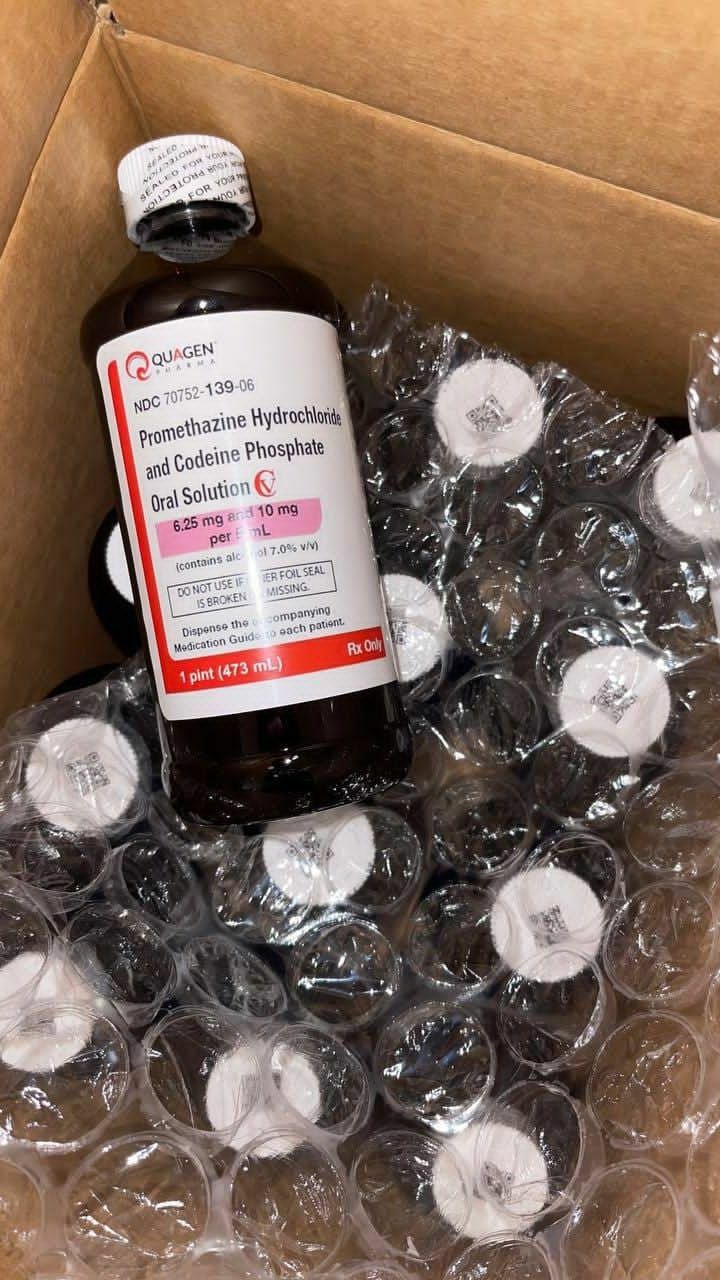
Buy Quagen Promethazine Hydrochloride And Codeine Phosphate Oral solution Online
Product Details Table
| Specification | Details |
|---|---|
| Brand | Quagen |
| Composition | Promethazine Hydrochloride (6.25 mg/5 mL) + Codeine Phosphate (10 mg/5 mL) |
| Concentration / Volume | Standard: 6.25 mg Promethazine HCl & 10 mg Codeine Phosphate per 5 mL; Various volumes available |
| Duration of Effects | Research observations typically note 4-6 hour activity window in study models |
| Indications / Usage Areas | Research applications: Analgesic combination studies, antitussive mechanisms, sedative effects, pharmacological interaction research |
| Administration Method | Oral solution for controlled laboratory administration |
| Shelf Life | 24-36 months from manufacture date (unopened, proper storage) |
| Storage Conditions | 20-25°C (68-77°F); protect from light and moisture; secure controlled access |
| Package Contents | Sealed bottle with child-resistant cap, product literature, Certificate of Analysis |
| Country of Origin | United States |
| Licensing / Certification | Manufactured in FDA-registered facility; cGMP compliant; Schedule V controlled substance for research |
| Safety Notes | RESEARCH CHEMICAL ONLY. Not for human consumption. Handle with PPE. Secure storage required. Potential CNS depression in research models. Follow all controlled substance regulations. |
$325.00 – $3,000.00
Buy it nowUnderstanding Quagen Promethazine Hydrochloride and Codeine Phosphate Oral Solution
Quagen Promethazine Hydrochloride and Codeine Phosphate Oral Solution represents a specialized pharmaceutical formulation designed for specific therapeutic applications. This solution combines two active pharmaceutical ingredients—promethazine hydrochloride, an antihistamine with sedative properties, and codeine phosphate, an opioid analgesic—to create a synergistic effect for targeted research and clinical study purposes.
Composition and Pharmacological Action
The solution contains precisely measured concentrations of promethazine hydrochloride (6.25 mg per 5 mL) and codeine phosphate (10 mg per 5 mL) in a stable oral suspension. Promethazine hydrochloride functions primarily as a histamine H1 receptor antagonist, producing calming and anti-nausea effects while potentially enhancing the analgesic properties of codeine. Codeine phosphate, an opioid agonist, is metabolized to morphine in the body, contributing to its studied analgesic effects. This combination creates a multifaceted compound valuable for controlled research environments investigating specific physiological responses.
Benefits and Research Applications
This formulation offers researchers several distinct advantages. The liquid oral solution allows for precise dosage administration, crucial in controlled experimental settings. The combination of mechanisms addresses multiple physiological pathways simultaneously, making it valuable for comparative studies in pain management research, cough reflex analysis, and sedative-effect investigations under laboratory conditions. The standardized concentration ensures consistency across research batches, while the oral delivery system mimics human administration routes for translational research value.
Proper storage maintains the solution’s chemical integrity for approximately 24-36 months from manufacturing date when kept in its original sealed container at controlled temperatures (typically 20-25°C/68-77°F). Protection from light, moisture, and temperature extremes preserves the active ingredients’ stability. Researchers should always verify expiration dates upon receipt and monitor solution clarity, as any precipitation or discoloration indicates potential degradation.
Safety and Responsible Usage Considerations
This product is intended exclusively for controlled laboratory research, clinical study, or educational purposes by qualified professionals in appropriate facilities. It is not for human consumption, personal use, or administration outside regulated research environments. Researchers must implement strict chain-of-custody documentation, secure storage in locked, access-controlled environments, and comply with all jurisdictional regulations governing Schedule V controlled substances.
Potential physiological effects observed in research models may include drowsiness, respiratory depression, gastrointestinal changes, and central nervous system depression. Appropriate personal protective equipment should be worn during handling, and facilities must have emergency protocols for accidental exposure. Disposal must follow hazardous pharmaceutical waste regulations specific to controlled substances.
Quality Assurance and Sourcing
Each batch undergoes rigorous analytical testing to verify concentration accuracy, purity, and stability. The solution is manufactured under current Good Manufacturing Practice (cGMP) standards in FDA-inspected facilities. Documentation including Certificate of Analysis, manufacturing records, and regulatory compliance statements accompanies qualified research orders.
Researchers sourcing this material should verify supplier credentials, regulatory compliance, and documentation transparency. As a specialized research chemical, acquisition is restricted to approved institutions, licensed researchers, and qualified professionals with appropriate regulatory approvals. For verified researchers seeking compliant sourcing, Health Aid Pharmacy maintains strict protocol adherence for qualified institutional clients.
Research Applications and Ethical Considerations
Primary research applications include pharmacological studies of combination analgesic effects, antitussive mechanism investigations, sedative properties analysis, and metabolic pathway research. The solution serves as a reference standard in analytical method development and comparative pharmaceutical studies.
Ethical research requires institutional review board approval where applicable, humane treatment of research models, and transparent reporting of findings. Researchers must maintain meticulous records of usage, storage, and disposal in compliance with the Controlled Substances Act and international research standards.
Customer Assurance and Research Support
Qualified research institutions consistently report satisfaction with the batch-to-batch consistency, documentation completeness, and chemical stability of this formulation when stored and handled according to specifications. The standardized concentration facilitates reproducible experimental conditions, while the oral delivery format enables specific administration route studies.
This product serves as a valuable tool for advancing pharmacological understanding when utilized responsibly within appropriate ethical and regulatory frameworks by qualified professionals in controlled research environments.
| Price Options | 16oz, 16oz case, 8oz, 8oz case |
|---|
| Ingredients | Skimmed milk powder, lactose, vegetable oils (palm, rapeseed, sunflower), palm kernel fat, calcium carbonate, calcium salts of phosphoric acid, vitamin C, ferrous sulphate, stabilizer: L (+) - lactic acid, vitamin D, riboflavin , vitamin A, potassium iodate. | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Nutritional values |
|
||||||||
| Available for age | 6-12 months, 12-24 months |












There are no reviews yet.