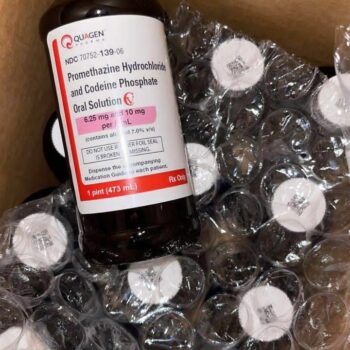
Product Description
For qualified researchers and institutions studying antitussive and antiemetic agents, access to consistent, pharmaceutical-grade compounds is essential. This Akorn-brand Promethazine Hydrochloride and Codeine Phosphate Oral Solution serves as a critical reference material for a range of in-vitro and analytical applications. Its specific combination of two active ingredients provides a multifaceted profile for scientific inquiry into their synergistic and individual effects.
What is Akorn Promethazine and Codeine Oral Solution?
This product is a precise pharmaceutical formulation originally developed as a prescription antitussive and antiemetic. As a research material, it provides a stable, consistent solution containing two distinct active compounds: promethazine hydrochloride, a phenothiazine derivative with antihistaminic and sedative properties, and codeine phosphate, an opiate alkaloid with central antitussive and mild analgesic effects. Its standardized concentration makes it invaluable for controlled laboratory studies where dosage accuracy is paramount.
How It Works: Mechanism of Action
In a research context, this combination allows for the study of dual-action pathways. Codeine Phosphate functions primarily as a prodrug, with a portion metabolized to morphine, where it acts as an agonist at mu-opioid receptors in the brain, suppressing the cough reflex center. Promethazine Hydrochloride complements this action as a competitive antagonist at histamine H1-receptors and possesses significant anticholinergic and central sedative effects. Together in experimental models, they provide a framework for studying combined suppression of cough reflex, modulation of nausea pathways, and central nervous system depression. Researchers can investigate the pharmacokinetics of their interaction, receptor binding affinities, and metabolic pathways.
Benefits and Advantages for Scientific Use
Choosing this Akorn-manufactured solution offers several key benefits for the research professional. First is the assurance of pharmaceutical-grade quality and consistency, as it is produced under strict cGMP standards, ensuring reliable and reproducible results across experiments. Secondly, its ready-to-use oral solution format eliminates compounding variables, saving valuable laboratory time and reducing potential preparation errors. Furthermore, it acts as a benchmark reference standard for developing and validating analytical methods (such as HPLC or LC-MS) aimed at detecting and quantifying these substances in other matrices. This makes it a staple in forensic, pharmacological, and toxicological research settings.
Safety & Responsible Usage Considerations
This product is provided solely for controlled laboratory research and analytical purposes. It is not intended for human or animal consumption, self-administration, or diagnostic use. Codeine phosphate is a Schedule II controlled substance in the United States and similarly regulated globally; its handling and procurement are subject to strict legal and regulatory controls. Researchers must possess the appropriate institutional licenses and DEA registrations (where applicable) to lawfully acquire, store, and utilize this material. Handling should involve appropriate personal protective equipment (PPE) in a secure, access-controlled laboratory environment. All local, state, and federal regulations must be strictly observed.
Longevity and Storage Life
To maintain the chemical integrity and stability of this solution for the duration of its research lifecycle, strict storage protocols must be followed. The product should be stored in its original, tightly sealed, light-resistant container. The optimal storage condition is at controlled room temperature, between 20°C to 25°C (68°F to 77°F), with excursions permitted between 15°C to 30°C (59°F to 86°F). It must be protected from light, excessive heat, and freezing. When stored under these prescribed conditions, the solution maintains its labeled potency and physical characteristics throughout its shelf life, as indicated on the packaging.
Ingredients / Composition
The oral solution contains two active ingredients per standard volumetric measure: Promethazine Hydrochloride (6.25 mg) and Codeine Phosphate (10 mg). The inactive vehicle typically includes ingredients such as citric acid, sodium citrate, glycerin, saccharin sodium, and purified water, creating a stable, palatable (in its medical context) solution. The exact formulation is consistent with the FDA-approved Akorn product, allowing for dependable compositional analysis.
Usage Areas for Research Purposes
In licensed and authorized research facilities, this solution has several defined applications. These include, but are not limited to: serving as a calibration standard in forensic toxicology for quantifying substance levels; studying the in-vitro metabolic pathways of codeine and promethazine; researching receptor binding kinetics for opioid and histaminic receptors; and developing wastewater analysis protocols for epidemiological studies on substance use. Sourced from a reputable manufacturer, this product underscores our commitment to supplying reference materials that meet the highest standards of traceability and quality. For our complete portfolio of analytical and research materials, please refer to our official resource at https://healthaidpharmacy.com/.














There are no reviews yet.