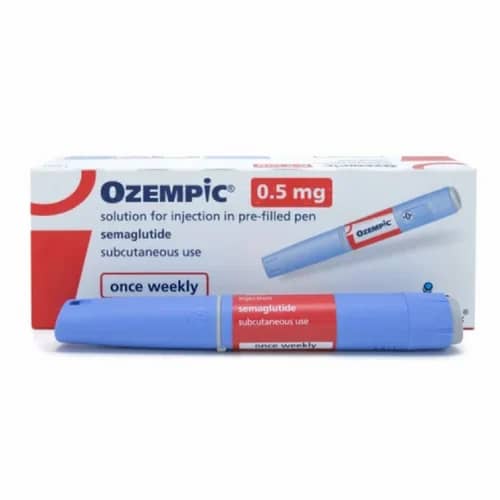
Understanding Ozempic (Semaglutide) Injection: A Comprehensive Overview for Researchers
Ozempic Semaglutide Injection For Sale is an antidiabetic medication used for the treatment of type 2 diabetes and an anti-obesity medication used for long-term weight management. It is a peptide similar to the hormone glucagon-like peptide-1 (GLP-1), modified with a side chain. It can be administered by subcutaneous injection or taken orally. It is sold under the brand names Ozempic . Semaglutide is a glucagon-like peptide-1 receptor agonist. The most common side effects include nausea, vomiting, diarrhea, abdominal pain, and constipation. It was approved for medical use in the US in 2017. In 2021, it was the 90th most commonly prescribed medication in the United States, with more than 8 million prescriptions. Semaglutide is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes.
The higher-dose formulation of semaglutide is indicated as an adjunct to diet and exercise for long-term weight management in adults with obesity (initial body mass index (BMI) ≥ 30 kg/m2) or who are overweight (initial BMI ≥ 27 kg/m2) and have at least one weight-related comorbidity. In March 2024, the FDA expanded the indication for semaglutide (Wegovy), in combination with a reduced calorie diet and increased physical activity, to reduce the risk of cardiovascular death, heart attack and stroke in obese or overweight adults with cardiovascular disease.
Eating disorders
Ozempic Semaglutide Injection For Sale and similar drugs, such as dulaglutide and liraglutide, have been used to treat binge eating disorder (BED), as they can successfully minimize obsessive thoughts about food and binging urges. Some users of these drugs have reported significant reduction in what is colloquially known as “food noise” (constant, unstoppable thoughts about eating despite not being physically hungry), which can be a factor of BED.
Adverse effects
Possible side effects include nausea, diarrhea, vomiting, constipation, abdominal pain, headache, fatigue, indigestion/heartburn, dizziness, abdominal distension, belching, hypoglycemia (low blood glucose) in people with type 2 diabetes, flatulence, gastroenteritis, and gastroesophageal reflux disease (GERD). It can also cause pancreatitis, gastroparesis, and bowel obstruction. The US FDA label for semaglutide contains a boxed warning for thyroid C-cell tumors in rodents. It is unknown whether semaglutide causes thyroid C-cell tumors, including medullary thyroid carcinoma, in humans
Semaglutide is a glucagon-like peptide-1 receptor agonist. The drug decreases blood sugar levels. The decrease is theorized to be caused by the mimicking of the incretin glucagon-like peptide-1 (GLP-1). It also appears to enhance growth of pancreatic beta cells, which are responsible for insulin production and release. Additionally, it inhibits the production of glucagon, the hormone that increases glycogenolysis (release of stored carbohydrate from the liver) and gluconeogenesis (synthesis of new glucose). It reduces food intake by lowering appetite and slowing down digestion in the stomach, helping reduce body fat.
Ingredients and Composition
The injection is a sterile, aqueous solution. Each milliliter contains the active ingredient, semaglutide, at the specified concentration. Inactive ingredients include disodium phosphate dihydrate, propylene glycol, phenol, and water for injections. The exact formulation is designed for stability and bioavailability via subcutaneous administration.
Primary Areas for Research Purposes
Ozempic semaglutide injection is utilized in preclinical and clinical research focusing on:
-
Type 2 Diabetes Mellitus pathophysiology and treatment models.
-
Obesity, weight management, and satiety signaling pathways.
-
Cardiovascular outcomes related to metabolic syndrome.
-
Non-alcoholic steatohepatitis (NASH) and fatty liver disease.
-
Potential cross-disciplinary applications in neurology or endocrinology.
We are committed to supporting the scientific community with reliable, high-grade research materials. For more information on our full catalog and compliance standards, please visit our official homepage at Healthaid Pharmacy. Our commitment is to empower discovery with integrity and quality, ensuring researchers have the trustworthy resources they need to push the boundaries of medical science.











There are no reviews yet.